Stay up-to date on the
latest blogs. Join our
newsletter today!
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Is Deionized Water The Same As Distilled Water?

Written by Team Ryze Chemie
11 mins read · Jun 08, 2024

Working with chemicals requires precision and care. Often, the water used in chemical processes can significantly affect results. Deionized and distilled water are two common types of purified water used in laboratories.
However, they are not the same. Many laboratory personnel often get confused between the two. Understanding the differences between them is critical to choosing the right one for your experiments.
This article will discuss the similarities and differences between deionized and distilled water and help you decide which one is more suitable for your work.
Understanding Water Purity
Water in nature contains minerals, dissolved gasses, and sometimes even microbes. These impurities can cause problems in scientific experiments. Pure water removes these impurities, but how pure is "pure"?
What is Water Purity?
Water purity means how clean the water is. Pure water only contains H₂O molecules. No other substances are present. But in reality, perfectly pure water is hard to find. Most water contains different impurities, such as dissolved minerals, organic matter, or gasses.
Why is Water Purity Important in the Lab?
Water impurities can interfere with chemical reactions. They can change results, cause errors, or even damage sensitive instruments. That's why we need to use water with the right purity level for each task in the lab.
Types of Impurities Found in Water
Water can contain many different types of impurities:
1) Dissolved Ions: These are minerals, salts, and heavy metals like calcium, magnesium, sodium, chloride, lead, and mercury. These ions can react with chemicals in our experiments and affect our results.
2) Organic Matter: This includes bacteria, viruses, algae, and dissolved organic compounds from decaying plant or animal matter. These can contaminate our samples and cause unwanted reactions.
3) Particulates: These are tiny bits of dust, debris, or insoluble matter that can clog filters, damage equipment, or interfere with optical measurements.
4) Dissolved Gasses: These are gasses like carbon dioxide, nitrogen, and oxygen that are dissolved in water. They can affect the pH of water and interfere with certain chemical reactions.
Total Dissolved Solids (TDS): A Measure of Water Purity
One way to measure water purity is by checking its TDS. TDS is the total amount of dissolved solids in the water. Higher TDS means more impurities in the water. We measure TDS in parts per million (ppm) or milligrams per liter (mg/L).
Different types of laboratory work need different levels of water purity. For example, making standard solutions or doing trace analysis might need very pure water with low TDS. But cleaning glassware or rinsing equipment might not need such high purity.
Now that we understand water purity, let's look at how we make two types of pure water.
How Deionized Water is Made
Deionized (DI) water is a cornerstone of laboratory work. It's a type of purified water that's had almost all of its mineral ions removed. This makes it ideal for experiments and procedures where even small amounts of impurities could cause problems. Let's break down how it's made and why it's so valuable.
How We Make Deionized Water: The Ion Exchange Process
The magic behind DI water production is ion exchange. This process uses special resins to swap out unwanted ions for hydrogen (H+) and hydroxide (OH-) ions. Here's the gist:
1) Cation Exchange: Positively charged ions (like calcium and magnesium) are attracted to a cation exchange resin. The resin releases hydrogen ions in their place.
2) Anion Exchange: Negatively charged ions (like chloride and sulfate) stick to an anion exchange resin. The resin releases hydroxide ions as replacements.
3) Pure Water: The released hydrogen and hydroxide ions combine to form H₂O – pure water!
Types of Deionization Systems
There are a couple of ways to set up this ion exchange process:
- Mixed-Bed Deionization: This is a single tank where both cation and anion exchange resins are mixed together. It's great for producing high-purity DI water in one step.
- Two-Bed Deionization: This uses two separate tanks, one for cation exchange and one for anion exchange. It's a bit more involved but can be more efficient for larger volumes of water.
Recharging the Resins
Over time, the resins fill up with unwanted ions and lose their effectiveness. But don't worry, we can recharge them! This involves flushing the resins with strong acids or bases to strip away the captured ions and restore their original state.
Advantages of Using DI Water in the Lab
- Purity: DI water gets rid of almost all ions, making it incredibly pure. This is crucial for experiments and procedures where even trace amounts of minerals could skew results.
- Cost-Effective: For labs that need a lot of purified water, deionization is a budget-friendly option compared to other methods like distillation.
- Different Grades: DI water comes in different purity levels (Type I, II, and III) to suit various lab needs. Type I is the purest, ideal for super sensitive work, while Type III is suitable for general lab cleaning.
Limitations to Keep in Mind
- Doesn't Remove Everything: While DI water is great at removing ions, it doesn't get rid of organic matter or tiny particles. If you need water that's free of those, you'll need additional purification steps.
- Bacterial Growth: DI water is essentially a blank slate, which means bacteria can thrive in it if it's not stored properly.
- Resin Exhaustion: Those handy ion exchange resins can't work forever. They'll eventually get exhausted and need to be recharged or replaced.
Deionization is one way to make pure water. Let's see how the other method, distillation, works.
How Distilled Water is Made
Distilled water is a mainstay in laboratories. It's a versatile solvent, a critical ingredient in solutions, and a cleaning agent for sensitive equipment. But how exactly is this purified water produced?
The Distillation Process: Step-by-Step
1) Heating: Ordinary tap water or purified water is placed in a distillation apparatus. This apparatus heats the water to its boiling point (100°C or 212°F).
2) Vaporization: As the water boils, it changes from a liquid to a gas (steam). This steam rises, leaving behind many impurities that don't vaporize at the same temperature.
3) Condensation: The steam travels to a cooling system. As it cools, it condenses back into a liquid. This collected liquid is the distilled water.
4) Collection: The distilled water drips into a clean collection vessel, ready for use or further purification.
Multiple Distillation for Extra Purity
Sometimes, one distillation cycle isn't enough. To achieve even higher purity, the distilled water can be distilled again. This repeated process removes more and more trace impurities.
Why Distilled Water Matters in the Lab
- Removes a Wide Range of Impurities: Distillation effectively removes many dissolved solids, ions, organic matter, and even some bacteria. This makes it a preferred choice for applications where even trace contaminants can interfere with reactions or experiments.
- Versatility: Distilled water is suitable for a wide range of laboratory tasks, from preparing reagents and buffers to rinsing glassware and cleaning equipment.
What Distillation Doesn't Remove
It's important to note that distillation is not a perfect process. While it removes many contaminants, some volatile organic compounds (VOCs) may vaporize along with the water and end up in the distilled product.
The Cost of Purity
Distillation comes with a few drawbacks:
- Energy Intensive: Boiling and cooling large volumes of water requires significant energy. This makes distillation a less environmentally friendly option compared to other purification methods.
- Cost: Due to the energy consumption and specialized equipment involved, distilled water tends to be more expensive than other types of purified water.
We have now seen two ways to make pure water. But which one is better?
Comparing Deionized and Distilled Water
Deionized (DI) and distilled water are two purified forms, each with unique strengths and weaknesses. Let's break down the differences so you can choose the right one for your experiments:
Head-to-Head Comparison:
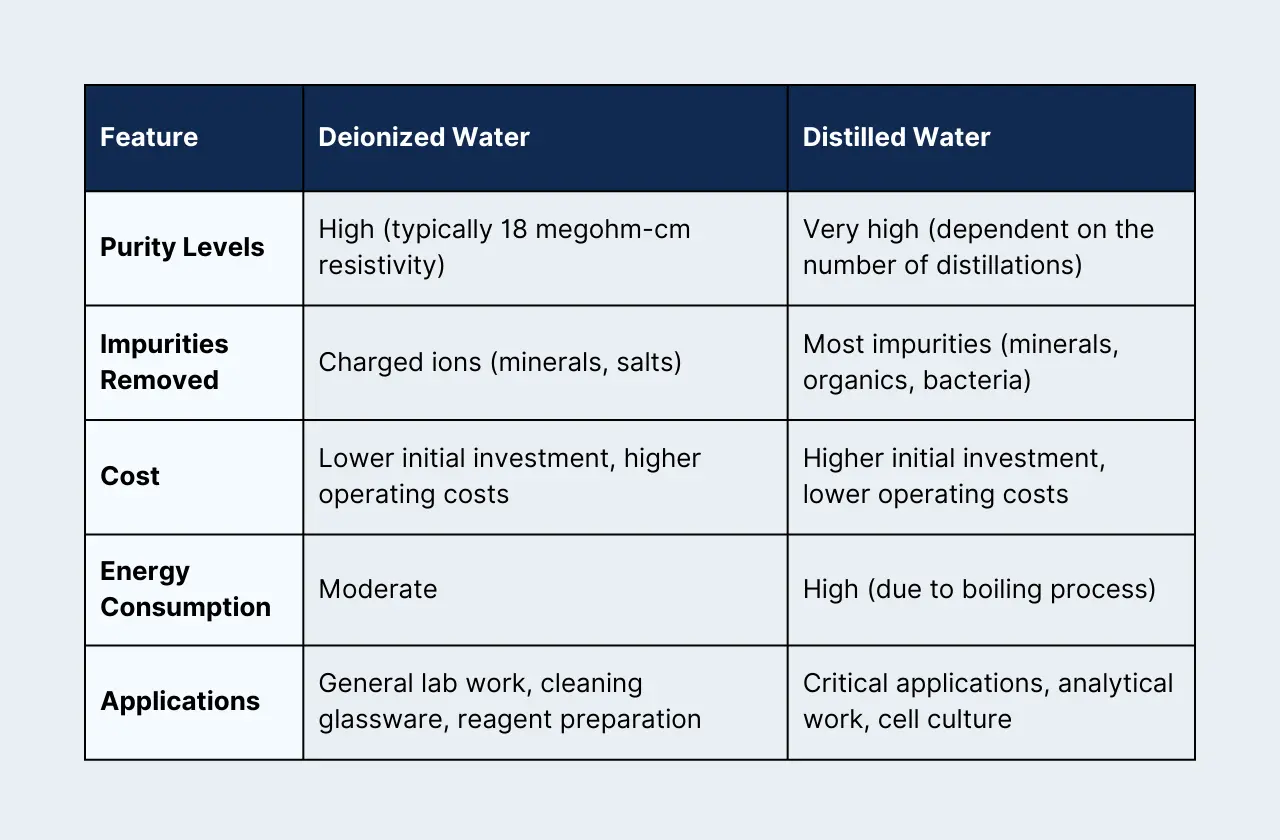
Why the Difference Matters:
- Purity: Both DI and distilled water remove impurities, but in different ways. DI water focuses on charged ions, while distillation removes a broader range of contaminants.
- Cost & Energy: DI water systems are cheaper upfront but require resin replacement and electricity to operate. Distillation is energy-intensive but often has lower ongoing costs.
- Applications: If your work demands extreme purity (like trace analysis or growing finicky cells), distilled water is often the better choice. DI water is a workhorse for everyday tasks.
Special Cases: Taking Purity to the Next Level
- Double-Distilled Water: This is distilled water that's been put through the boiling and condensation process twice. It's incredibly pure and ideal for the most sensitive experiments.
- Polished DI Water: Some labs add additional filters to their DI systems (like carbon or UV) to remove any trace organics or bacteria that might remain. This provides an extra layer of confidence for critical work.
Choosing Your Champion: Questions to Ask Yourself
1) What's my budget? If cost is a major factor, DI might be your starting point.
2) How pure does my water need to be? Distilled water wins for ultimate purity, especially the double-distilled variety.
3) What am I using the water for? Everyday tasks like cleaning glassware can get by with DI water. Critical applications like HPLC or cell culture might demand distillation.
4) Do I need to remove specific contaminants? Think about whether charged ions, organics, or bacteria are your main concerns.
With a clear understanding of both types of water, let's see how to choose the right one for your lab work.
Choosing the Right Water for Your Lab
The right choice between DI water and distilled water depends on what you are using it for. Some lab tasks need one, while others need the other:
1) Guidelines Based on Application:
- Critical analytical work (e.g., HPLC, trace element analysis): You need the purest water possible. This water is often referred to as Type I or ultrapure water. It undergoes rigorous purification processes to remove impurities like ions, organics, bacteria, and even dissolved gasses.
- General lab work (e.g., reagent preparation, glassware rinsing): Type II water usually suffices for these tasks. It's purer than tap water but doesn't require the same level of purification as Type I.
- Equipment cleaning and sterilization: Type III water is generally suitable. It's produced through processes like reverse osmosis and can remove most contaminants.
2) Water Quality Standards:
- Reference relevant ASTM and ISO standards: These organizations have established guidelines for different water purity levels. Check these standards to ensure your water meets the requirements for your intended use.
- Importance of regular testing and monitoring of water quality: Even with a high-quality purification system, it's essential to test your water regularly. This helps identify any issues and ensures that your water quality remains consistent.
3) Cost Considerations:
- Balance purity needs with budget constraints: High-purity water can be expensive to produce. Assess your lab's needs and determine the minimum purity level required for each application.
- Explore in-house vs. purchasing options: You can purchase purified water from commercial suppliers or produce it in-house using a purification system. Compare costs and weigh the pros and cons of each option.
Pro Tips for Lab Water Management
- Storage matters: Store purified water in clean containers to prevent contamination.
- Label clearly: Label all containers with the water type, purity level, and date of production.
- Train your team: Ensure everyone in the lab understands the importance of using the correct water for each task.
- Invest in quality: A good water purification system is an investment in the accuracy and reliability of your lab's work.
Conclusion
In conclusion, deionized and distilled water are distinct types of purified water, each with unique properties and applications. While both remove impurities, their methods differ. Distillation involves boiling and condensation, while deionization uses ion exchange resins.
Choose distilled water when you need water free of microbes and volatile organic compounds. Opt for deionized water to eliminate ions and dissolved solids.
Remember, neither is inherently superior; the "best" choice depends on your specific laboratory needs. Consult safety guidelines and protocols when deciding which water to use for your experiments and processes.
Frequently Asked Questions (FAQs)
1) Can I use tap water for general lab work?
Tap water often contains impurities that can affect your experiments. It's best to use purified water to ensure accurate results.
2) How often should I test my water quality?
The frequency of testing depends on the type of work you're doing and the quality of your purification system. A good rule of thumb is to test at least once a month, but more frequent testing may be necessary for critical applications.
3) What's the difference between distilled and deionized water?
Distillation removes impurities by boiling the water and collecting the condensed vapor. Deionization removes ions using specialized resins. Both methods produce purified water, but the choice depends on your specific needs.
Latest Blogs