Stay up-to date on the
latest blogs. Join our
newsletter today!
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Is Boiled Water The Same As Distilled Water?

Written by Team Ryze Chemie
10 mins read · Jun 04, 2024

Laboratory personnel often use water in their work. They might find it confusing to choose the right type of water for their work. Tap water is readily available but it contains many impurities.
We can remove some impurities from tap water by boiling it. But boiling does not remove all impurities from tap water. This is where distilled water is better than boiled water. Distilled water is the purest form of water.
In this article, we will define boiled water and distilled water. We will also discuss the differences between the two.
Defining Boiled Water and Distilled Water
Let's break down the difference between two types of water you often see in the lab: boiled water and distilled water. It's a simple concept, but super important for your work.
What is Boiled Water?
- How it's made: We make boiled water by heating it until it bubbles and steams. You know, the good ol' kettle method!
- Why we boil it: Boiling water zaps away most of the tiny living things that could mess with our experiments. It's like a quick clean-up crew for your water!
- What's still in it: While boiling kills most bacteria and viruses, it doesn't remove the stuff that's dissolved in the water. So, things like minerals and salts are still hanging around.
What is Distilled Water?
- How it's made: Distilled water takes things a step further. First, we boil the water just like before. Then, we catch the steam that rises up and cool it down. As it cools, it turns back into water, but now it's in a separate container.
- Why we distill it: This steam-catching trick leaves behind nearly all the impurities. We're talking about a super-pure form of water!
- What's in it (or not!): Distilled water is the closest you can get to pure H₂O using simple lab methods. That's why it's so handy for experiments where you need to be extra sure about what's in your water.
Now that we understand what each type is, let's see how they compare.
Is Boiled Water the Same as Distilled Water?
Boiled and distilled water are common in laboratories, but they are not interchangeable. Knowing their differences is crucial for accurate experiments and equipment care. Let's break down the key distinctions:
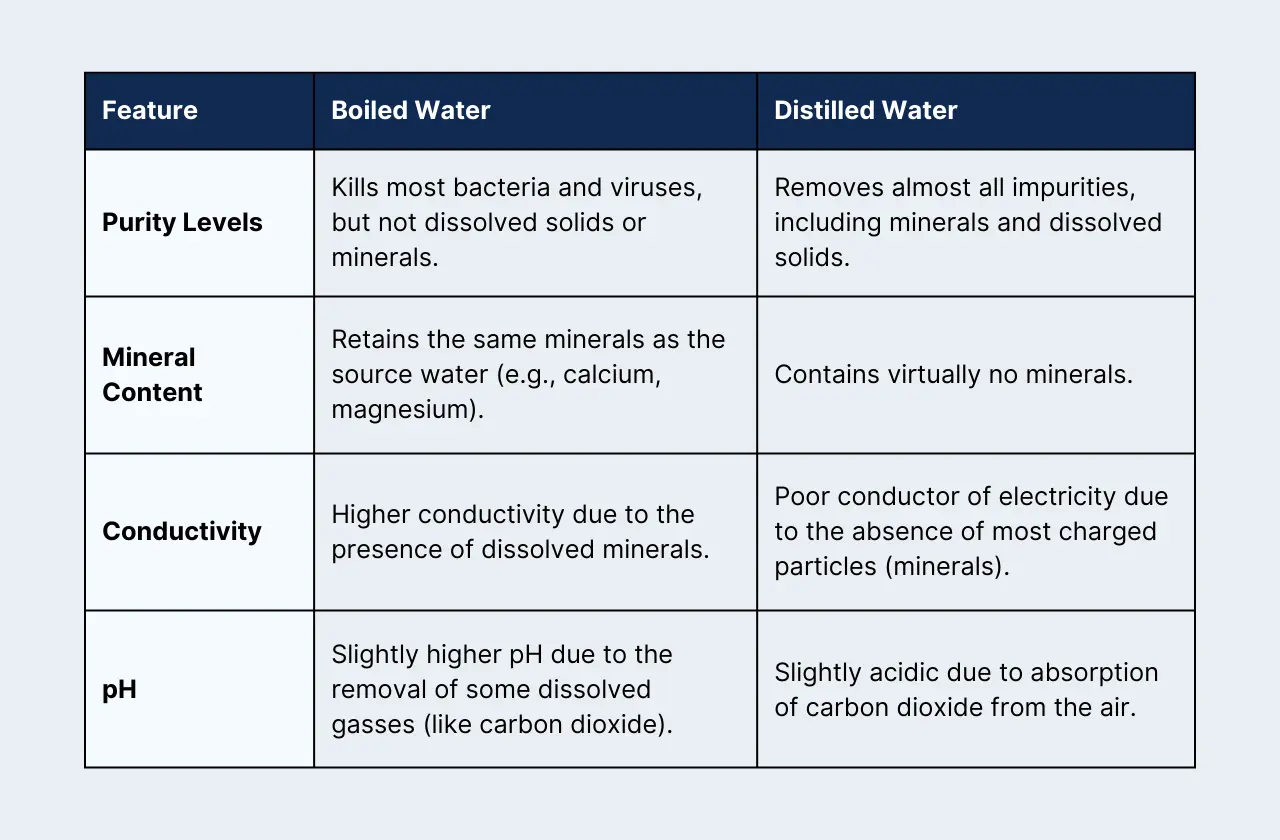
1) Purity Levels:
- Boiled Water: Boiling water kills most bacteria and viruses, making it safer to drink. However, it doesn't remove dissolved solids, minerals, or other impurities. Think of it like a cleaned-up version of your tap water, not a pristine one.
- Distilled Water: This water is the VIP of purity. Distillation involves boiling the water and then collecting the condensed steam. This process leaves behind almost all impurities, resulting in ultra-pure water ideal for sensitive lab work.
2) Mineral Content:
- Boiled Water: Your boiled water will have the same minerals as your tap water. These can include calcium, magnesium, and others, varying based on your location's water source.
- Distilled Water: Distillation strips out nearly all minerals. This can be great for preventing mineral buildup in equipment, but it's not the best for drinking regularly as we need those minerals!
3) Conductivity:
- Boiled Water: Minerals in water act like tiny charged particles, allowing electricity to flow. Boiled water, with its minerals intact, will have higher conductivity. This matters when working with electronics or solutions where electrical properties are important.
- Distilled Water: Since most charged particles are removed, distilled water is a poor conductor of electricity. It's like a roadblock for electrical current, making it useful in applications where conductivity needs to be minimized.
4) pH:
- Boiled Water: Boiling water can slightly raise its pH. This is because some dissolved gasses, like carbon dioxide (which makes water slightly acidic), are driven off.
- Distilled Water: Freshly distilled water is slightly acidic because it quickly absorbs carbon dioxide from the air. This is important to note for pH-sensitive experiments.
These differences have important effects on how we use water in the lab.
Implications for Laboratory Work
Why does this matter in the lab? Different types of water can affect our results. This section explains how. It also tells us why choosing the right water is key to good science:
1) Reagent Preparation:
Distilled water is the cornerstone of accurate reagent preparation. Tap water often contains impurities like minerals, chlorine, and dissolved organic matter. These can react with chemicals in unpredictable ways, leading to inaccurate results or even ruining experiments.
For instance, trace amounts of iron in tap water can interfere with colorimetric assays, while calcium can cause precipitation in solutions. Distilled water eliminates these risks, ensuring reagents perform as expected.
Specific examples of reactions sensitive to impurities:
- Preparation of buffers for pH control
- Dilution of standard solutions for calibration
- Dissolving reagents for spectrophotometric analysis
2) Analytical Procedures:
Many analytical techniques demand high-purity water to guarantee accurate and reliable results. Distilled water minimizes the risk of contamination from dissolved ions, organic compounds, or particulate matter. This is crucial in techniques like:
- High-Performance Liquid Chromatography (HPLC): Impurities can interfere with separation, detection, and quantification of analytes.
- Spectroscopy (UV-Vis, IR, etc.): Dissolved substances can absorb or scatter light, leading to erroneous readings.
- Electrochemical analysis: Ionic impurities can alter conductivity and electrode potentials, affecting measurements.
Using distilled water in these techniques ensures that your results reflect the true properties of the sample, not artifacts from the water used.
3) Cleaning and Rinsing:
Distilled water is an excellent choice for cleaning laboratory glassware and equipment. It prevents mineral deposits that can form when tap water evaporates, leaving behind residues that might interfere with subsequent experiments.
Even after cleaning with detergents, a final rinse with distilled water is essential. This removes any traces of cleaning agents and ensures that your glassware is pristine for the next use.
Let's look at some specific situations where the right choice of water matters.
When to Use Each Type?
In the lab, we do many different tasks. Some tasks require boiled water. Some need distilled water. This section gives clear examples of when to use each one:
Boiled Water:
Boiled water is your go-to for everyday tasks in the lab. Why? It's simple, quick, and gets the job done for most general cleaning needs.
- General Cleaning: Think countertops, the outside of equipment, or even your hands. Boiled water removes dirt, dust, and many common germs without the need for fancy chemicals.
- Non-Critical Equipment: Some lab tools aren't used for the most sensitive experiments. That old hot plate? Give it a wipe-down with boiled water. It's good enough.
- Some Biological Work: Need to rinse out a container that holds cells, but the minerals in water won't mess things up? Boiled water is a safe bet.
Important Note: Boiled water is NOT sterile. It may still contain some impurities or heat-resistant bacteria.
Distilled Water:
Distilled water is like the VIP of the water world. It's been through a special process to remove almost all impurities, including minerals and most microbes. This makes it perfect for work where precision is key.
- Making Solutions: Imagine baking a cake and the recipe calls for accurate measurements. That's what it's like making chemical solutions. Distilled water ensures your measurements are accurate and won't throw off your results.
- Critical Tests: Think of those experiments where even tiny changes matter. Distilled water is your best friend here. No unwanted minerals or other stuff to mess up the results.
- Squeaky Clean Glassware: The last rinse with distilled water gives your glassware a sparkling finish. No spots or streaks left behind, so your instruments stay accurate and reliable.
Tip: Label your distilled water bottles clearly. You don't want to accidentally grab regular water when you need the pure stuff!
Before you choose your water, remember that safety is always a factor.
Safety Considerations
Working with chemicals in the lab requires attention to detail and a strong focus on safety. Even something as seemingly simple as distilled water can pose risks if not handled correctly. Let's break down some essential safety considerations:
1) Storage of Distilled Water:
Distilled water is like a blank canvas – it's super pure. Here's how to keep it that way:
- Clean Containers: Always store distilled water in containers that are clean and specifically meant for this purpose. This can be glass or special plastic containers that won't leach chemicals.
- Tightly Sealed: Keep the container's lid on tight. This prevents dust, lab fumes, or other airborne particles from getting in.
- Label It: Always label the container clearly with "Distilled Water" and the date it was prepared or purchased.
- Cool & Dark Storage: Store your distilled water in a cool, dark place away from direct sunlight. This helps to keep it stable and prevents unwanted reactions.
- Check for Changes: Before each use, quickly check your distilled water. If it looks cloudy, has particles, or smells off, don't use it. Get a fresh supply.
Why All the Fuss? Contaminants in your distilled water can mess up your experiments, reactions, or equipment. It's worth taking the extra steps to get accurate results!
2) Deionized Water:
Deionized (DI) water is also super pure, but in a different way. It has almost all of its ions removed.
- Similar Storage: The same storage rules for distilled water mostly apply to deionized water.
- When to Use DI: Deionized water is often used for applications where even tiny amounts of ions can cause problems, like when preparing analytical standards or rinsing delicate equipment.
- Fresh is Best: Deionized water doesn't stay pure forever. Use it shortly after it's made for best results.
Key Point: Distilled water is great for most lab tasks, but deionized water has its niche when ion contamination is a big concern.
3) Lab-Specific Guidelines:
Every lab is different. The chemicals you use, the type of work you do – it all impacts how you should handle water.
- Read Your Safety Manual: Your lab's safety manual is your guidebook. It'll tell you exactly how they want distilled and deionized water stored and used.
- Ask Your Supervisor: If you're not sure about anything, don't guess. Ask your supervisor or a senior colleague for help. Safety always comes first.
- Stay Updated: Lab procedures can change. Keep up with any updates or new guidelines that come out.
Remember: Even something as simple as water can be a hazard if not handled properly. By following these tips and your lab's specific rules, you'll be working safely and getting accurate results.
Conclusion
In conclusion, boiled water is water heated to its boiling point, killing most microbes. It still contains minerals and other impurities. Distilled water, on the other hand, is purified water that has gone through vaporization and condensation, removing nearly all impurities.
Understanding these differences is crucial for lab work. Use boiled water for general cleaning and non-critical tasks. Use distilled water for experiments, reagent preparation, and applications that require high purity. Always refer to your lab's specific protocols for the appropriate water type.
Latest Blogs