Stay up-to date on the
latest blogs. Join our
newsletter today!
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
How does HPLC work? Step-by-step (2024)

12 mins read · May 09, 2024

Analyzing compounds accurately poses a challenge in labs. Operators often face difficulties in separating and identifying individual substances within mixtures. High-Performance Liquid Chromatography (HPLC) stands as a reliable solution.
However, understanding its operation remains elusive for many. Step-by-step comprehension of HPLC's functioning is crucial for efficient utilization in chemical analysis. In this article, we unravel the intricacies of HPLC operation, breaking down each stage into simple terms.
It is essential for laboratory personnel to gain a fundamental understanding of HPLC prior to using it to investigate compounds accurately and ensure reliable results.
Core Principles of HPLC
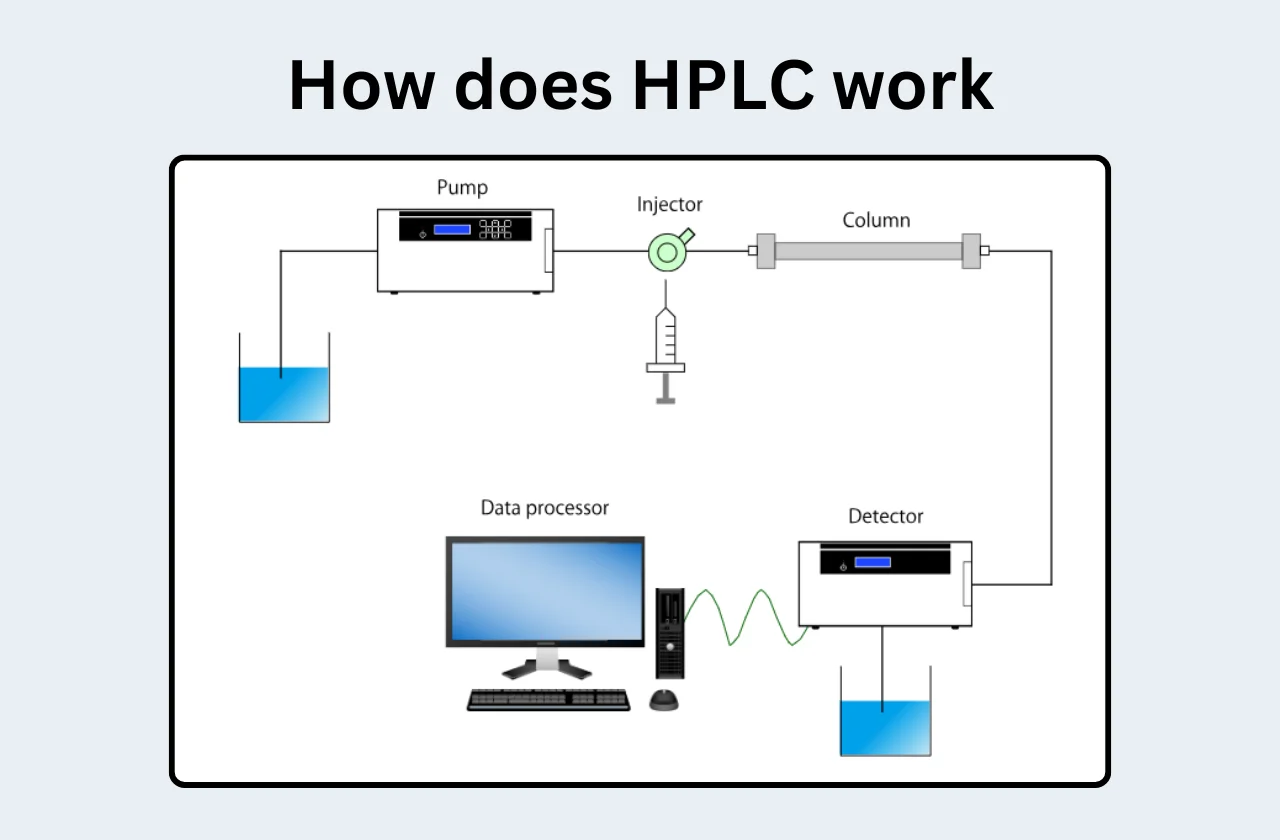
High-performance liquid chromatography (HPLC) is a powerful analytical technique used to separate, identify, and quantify components within a mixture. It achieves this by exploiting the differing interactions of sample compounds with two key phases: the mobile phase and the stationary phase. Understanding the core components of an HPLC system and their roles is essential for successful analysis.
Fundamental Components of an HPLC system:
Solvent Reservoir:
The solvent reservoir holds the mobile phase, a liquid or solvent mixture that continuously flows through the HPLC system. The mobile phase plays a crucial role in separating sample components. Different solvents have varying polarities, which influence their interaction with the stationary phase and ultimately affect the separation of analytes. Common solvents used in HPLC include:
- Acetonitrile (ACN): Polar aprotic solvent, good for dissolving nonpolar analytes.
- Methanol (MeOH): Polar protic solvent, miscible with water and organic solvents, often used in combination with ACN.
- Water (H2O): Universal solvent, commonly used with modifiers like organic acids or buffers to adjust pH.
The choice of solvent(s) depends on the specific analytes and the desired separation.
Pump:
The pump is the heart of the HPLC system. It delivers the mobile phase at a constant and high pressure (up to 400 atm) through the column. Consistent flow rate is critical for achieving optimal separation and maintaining reproducibility. Factors to consider when selecting a flow rate include:
- Column dimensions: Smaller columns require lower flow rates.
- Particle size of the stationary phase: Smaller particles require lower flow rates.
- Resolution desired: Higher resolution often requires slower flow rates.
- Analysis time: Faster flow rates decrease analysis time but may compromise resolution.
Selecting the appropriate flow rate involves balancing separation efficiency with analysis speed.
Injector:
The injector introduces a precise volume of the sample solution into the mobile phase stream. Several injection methods exist, with loop injection being a common technique. In loop injection, a defined volume of sample is loaded into a loop. The injector valve then switches, directing the sample onto the head of the column, where it is carried by the mobile phase.
Column:
The column is the separation chamber where the magic of HPLC happens. It houses the stationary phase, a packed bed of microscopic particles. The mobile phase carries the sample components through the column, where they interact with the stationary phase to varying degrees. This interaction determines how long each component spends in the column, resulting in their separation.
There are different types of columns based on the separation mechanism:
- Reversed-phase (RP): Most widely used, separates analytes based on hydrophobicity (water-hating). Analytes with higher hydrophobicity interact more strongly with the stationary phase and elute later.
- Normal-phase: Separates based on polarity. Analytes with higher polarity interact more with the polar stationary phase and elute later.
- Ion-exchange: Separates charged molecules based on their interaction with charged functional groups on the stationary phase.
The selection of the column type depends on the physicochemical properties of the analytes being separated.
Detector:
The detector monitors the mobile phase exiting the column and generates a signal based on the presence and amount of analytes eluting. Common detector types include:
- Ultraviolet-visible (UV-Vis): Detects analytes that absorb UV or visible light. Simple and versatile, but limited to analytes with chromophores (light-absorbing groups).
- Mass spectrometry (MS): Highly specific and sensitive detectors can identify and quantify analytes based on their mass-to-charge ratio. More complex and expensive than UV-Vis.
The choice of detector depends on the specific needs of the analysis, considering factors like sensitivity, selectivity, and compatibility with the mobile phase.
Data Acquisition System:
The data acquisition system controls the HPLC instrument and collects the signal from the detector. This information is displayed as a chromatogram, a graph showing peaks corresponding to the separated analytes. The area under each peak is proportional to the amount of the corresponding analyte. The data acquisition system allows for the analysis of peak retention times, peak areas, and the calculation of analyte concentrations.
Recording and analyzing data is crucial for interpreting the results of an HPLC experiment. By studying the chromatogram, analysts can identify and quantify the components in a mixture and assess the success of the separation.
Now that we've covered the core principles, let's move on to a step-by-step analysis of HPLC.
Step-by-Step HPLC Analysis
High-performance liquid chromatography (HPLC) is a powerful analytical technique for separating and identifying components in a mixture. Obtaining accurate and reliable results requires careful attention to each step of the analysis, from sample preparation to data interpretation.
Sample Preparation:
Proper sample preparation is crucial for successful HPLC analysis. Here's why:
- Filtration: Solid particles in the sample can clog the column and damage the instrument. Filtration removes these particles, ensuring smooth flow and protecting the system.
- Dilution: Highly concentrated samples can overload the column, leading to poor peak shapes and inaccurate quantification. Dilution reduces the concentration to an appropriate level for analysis.
Solvent selection is critical for effective sample preparation. Consider:
- Analyte solubility: The chosen solvent must effectively dissolve the target analytes. Experiment with different solvents to find the best one for your specific sample.
- Compatibility: The solvent should not react with the analytes or degrade the sample matrix. Consult safety data sheets (SDS) for compatibility information.
Mobile Phase Preparation:
The mobile phase is the solvent mixture that continuously flows through the HPLC system, carrying the sample through the column. It plays a vital role in separating the analytes:
- Separation: The mobile phase interacts with the stationary phase in the column and the analytes in the sample. This interaction affects how quickly each analyte travels through the column, leading to their separation.
- Polarity: The polarity of the mobile phase significantly influences separation. A more polar mobile phase interacts more strongly with polar analytes, causing them to elute (exit the column) slower than less polar analytes.
Common mobile phase modifiers like acids and bases can be added to fine-tune the interaction between analytes and the column. These modifiers can:
- Increase or decrease the ionization state of analytes, affecting their affinity for the stationary phase.
- Adjust the overall polarity of the mobile phase, influencing analyte separation.
Column Selection:
The HPLC column houses the stationary phase, a critical element for separating analytes. Choosing the right column is essential:
- Target Analytes: The column chemistry should be compatible with the target analytes. For example, reversed-phase columns are commonly used for separating non-polar and moderately polar analytes.
- Separation Mechanism: Different column chemistries offer distinct separation mechanisms based on analyte properties like size, polarity, or charge. Understanding the analytes and desired separation mechanism guides column selection.
Method Development and Optimization:
Developing an optimized HPLC method involves strategically adjusting various parameters to achieve the best possible separation for your specific analytes. Key parameters for optimization include:
- Solvent composition: The ratio of solvents in the mobile phase can be fine-tuned to improve peak resolution and separation.
- Flow rate: Flow rate adjustment affects how quickly analytes move through the column. An optimal flow rate balances separation efficiency with analysis time.
- Gradient elution: A gradient elution program gradually changes the mobile phase composition during the analysis. This technique can be helpful for separating analytes with a wide range of polarities.
Sample Injection and Separation:
Sample injection introduces the prepared sample into the HPLC system. The injection volume and technique can significantly impact:
- Peak shape: An appropriate injection volume ensures well-defined peaks for accurate analysis.
- Resolution: Precise injection minimizes band broadening, which can lead to overlapping peaks and hinder separation.
Inside the column, separation occurs based on the differential interactions between analytes and the stationary phase. Analytes with a stronger affinity for the stationary phase move slower through the column compared to those with weaker interactions. This difference in interaction times leads to the separation of analytes as they exit the column at different times.
Detection and Data Analysis:
The detector in an HPLC system identifies and quantifies the separated analytes. Common detectors include ultraviolet (UV) detectors that measure analyte absorbance at specific wavelengths.
For quantitative analysis, calibration standards with known concentrations are used. By comparing the peak area of the analyte to the peak area of the standard, the concentration of the analyte in the sample can be calculated.
Data analysis software is essential for interpreting the information obtained from the detector. The software displays the chromatogram, which is a plot of detector signal versus time. Key data points include:
- Retention times: The time it takes for each analyte to reach the detector, providing a characteristic fingerprint for identification.
- Peak areas: The area under each peak in the chromatogram is proportional to the amount of analyte present, allowing for quantification.
By carefully considering each step of the HPLC analysis process, from sample preparation to data interpretation, laboratory personnel can ensure accurate, reliable, and efficient separation of components in complex mixtures.
With the analysis process understood, let's address common issues that may arise and how to troubleshoot them.
Troubleshooting Common HPLC Issues
Despite careful preparation, HPLC experiments can encounter various issues. In this section, we'll discuss some of the common problems you may face, such as baseline drift, peak broadening, and retention time shifts, along with practical troubleshooting strategies to resolve them:
Peak Broadening
Broadened peaks can obscure target peaks and make quantification difficult. Here are some common causes and solutions for peak broadening:
- Injection volume overload: Inject too much sample, and the analyte molecules overload the column. This disrupts the separation process, leading to broader peaks. Reduce the injection volume and re-inject the sample.
- Incorrect mobile phase composition: The mobile phase is responsible for separating analytes. An unsuitable mobile phase composition can cause analytes to elute too quickly or slowly, resulting in broader peaks. Review your method and adjust the mobile phase composition (e.g., solvent ratios, pH) based on analyte properties.
- Flow rate issues: Flow rate directly affects peak shape. A flow rate that is too high can lead to broader peaks due to less interaction between analytes and the stationary phase. Conversely, a flow rate that is too low can cause excessive band broadening. Check your flow rate settings and adjust them according to the established method.
- Column problems: A dirty or damaged column can cause peak broadening. Contaminants can accumulate on the column over time, hindering analyte separation. Regularly clean the column according to the manufacturer's instructions. If cleaning doesn't help, consider replacing the column.
Poor Resolution
Poor resolution means analytes elute too close together, making them difficult to distinguish. Here's how to troubleshoot:
- Mobile phase selection: The mobile phase plays a crucial role in separating analytes. Choose a mobile phase that interacts differently with the analytes, allowing for better separation. Experiment with different solvent combinations or adjust the pH of the mobile phase.
- Gradient optimization: In gradient elution, the mobile phase composition changes over time. An improperly designed gradient can lead to poor resolution. Review your gradient profile and adjust the gradient slope or solvent ratios to achieve better separation between analytes of interest.
- Column selection: The stationary phase in the column interacts with analytes. Using the wrong column chemistry can result in poor resolution. Consider using a different column with a stationary phase that offers better selectivity for your analytes.
Ghost Peaks
Ghost peaks are extraneous peaks that appear in the chromatogram but don't correspond to any components in the sample. These can complicate data analysis. Here are some potential causes and solutions:
- Mobile phase impurities: Contaminants in the mobile phase can elute from the column and show up as ghost peaks. Prepare a fresh mobile phase with high-purity solvents and consider filtering the mobile phase before use.
- System contamination: Dirty HPLC lines, injectors, or detectors can introduce contaminants that show up as ghost peaks. Flush the system with appropriate solvents to remove any accumulated contaminants.
- Sample carryover: Sample components can remain in the system after an injection, causing them to appear in subsequent injections as ghost peaks. Ensure proper rinsing of the injection system between injections. Consider increasing the wash volume or using a stronger wash solvent.
General Troubleshooting Tips
- Review your method: Double-check your HPLC method parameters like flow rate, mobile phase composition, injection volume, and column selection. Ensure they are appropriate for the analytes you are analyzing.
- Maintain your instrument: Regularly clean and maintain your HPLC system according to the manufacturer's instructions. This includes replacing frits, seals, and filters as needed.
- Use a system suitability test: Run a system suitability test before injecting your samples. This helps ensure the HPLC system is performing optimally and can generate reliable data.
- Keep a logbook: Document your observations, including peak shapes, retention times, and any changes made to the method. This will help you identify trends and troubleshoot issues more effectively.
By following these tips and systematically addressing potential causes, you can effectively troubleshoot common HPLC problems and ensure your analyses are accurate and reliable. Remember, consulting your instrument manual and the manufacturer's technical support can also be valuable resources when troubleshooting specific issues with your HPLC system.
Conclusion
In conclusion, High-Performance Liquid Chromatography (HPLC) separates compounds in a liquid mixture. The sample passes through a column filled with packing material. Components interact differently with the stationary phase. The mobile phase pushes the sample through the column. As components separate, they exit the column at different times. A detector identifies and measures each component. Retention time indicates the time taken for each compound to exit the column. HPLC's efficiency depends on factors like column type and mobile phase composition. Regular maintenance ensures accurate results. Understanding HPLC's step-by-step process is vital for precise chemical analysis in laboratories.
Latest Blogs