Stay up-to date on the
latest blogs. Join our
newsletter today!
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Is RO Water The Same As Distilled Water

9 mins read · May 10, 2024

Is RO water identical to distilled water? This question frequently arises amongst laboratory personnel dealing with chemical substances. Understanding the subtle but critical differences among the two types of water is essential for unique experimentation and analysis.
RO water, produced through reverse osmosis, undergoes filtration to eliminate impurities; however, it may not dispose of all contaminants. Distilled water, on the other hand, undergoes a rigorous technique of vaporization and condensation, resulting in notably pure water.
With the help of this article, lab experts can make clear the variations between RO and distilled water, ensuring accuracy and reliability in their experiments.
Understanding Water Purification Methods
Water purification methods are vital in ensuring the integrity of lab experiments. By comprehending these techniques, lab personnel could make informed selections regarding water quality and suitability for numerous applications:
Reverse Osmosis (RO)
The RO technique utilizes strain to pressure water via a semipermeable membrane. This membrane has pores that permit water molecules to pass through; however, they entice large contaminants on the alternative facet. Imagine a strainer that we could water bypass through; however, it stops rice grains. In RO, the "rice grains" are various impurities inside the water.
RO removes a wide variety of contaminants, including:
- Dissolved salts: RO is quite powerful at getting rid of dissolved salts, including sodium and chloride, common additives of table salt. This makes RO a helpful method for demineralizing water.
- Minerals: Similar to dissolved salts, RO can remove many minerals like calcium and magnesium that contribute to water hardness.
- Bacteria: The organism's size is much larger than water molecules. So, the semipermeable membrane successfully blocks bacteria from passing through the purified water.
- Organic compounds: RO can remove a few organic compounds, relying on their size and molecular structure. Smaller organic molecules may slip through the membrane, while larger ones are typically rejected.
Distillation
Distillation is a process that purifies water by boiling it to create vapor and then condensing the vapor back into liquid form. This process can be visualized like this: Imagine boiling a pot of dirty water and collecting the clean steam in another container. The condensed steam is purified water.
Distillation removes a wide range of contaminants, including:
- Dissolved solids: Distillation removes all dissolved solids, including salts and minerals, because they are left behind when the water evaporates.
- Minerals: As with dissolved solids, minerals are nonvolatile and remain in the boiling flask during distillation.
- Bacteria: The high temperature of boiling kills bacteria present in the water.
- Viruses: Similar to bacteria, distillation effectively eliminates viruses due to the boiling temperature.
- Volatile organic compounds: Most organic compounds are volatile, meaning they evaporate easily. During distillation, these organic compounds vaporize with the water and are not condensed back into the purified water.
Next, we'll dissect the key differences between RO and distilled water, particularly focusing on their relevance in laboratory settings.
Key Differences Between RO and Distilled Water for Lab Use
Choosing the right type of purified water is crucial for many laboratory experiments. Both reverse osmosis (RO) and distilled water offer high levels of purity, but they differ in their removal of specific contaminants. Understanding these differences is essential for selecting the best water for your research needs.
1) Mineral Content
Distilled Water: Distillation removes all dissolved minerals from water. This process involves boiling the water and collecting the steam, which condenses into pure water vapor. Since minerals don't readily vaporize, they are left behind in the boiling chamber. The resulting distilled water is essentially deionized water, meaning it lacks charged ions present in dissolved minerals.
RO Water: RO purification uses a semi-permeable membrane that allows water molecules to pass through but traps most dissolved minerals. The effectiveness of mineral removal depends on the type of RO membrane used. Some high-rejection membranes can remove a very high percentage of minerals, approaching the purity of distilled water. However, other RO membranes may allow a small amount of minerals to pass through.
2) Conductivity
Distilled Water: Due to the absence of dissolved ions, distilled water has very low electrical conductivity. Conductivity is a measure of water's ability to conduct electricity, and ions play a significant role in this process. Since distilled water lacks these charged particles, it conducts electricity very poorly. This low conductivity is a key advantage when working with solutions where even slight variations in ionic content can affect results.
RO Water: RO water may have slightly higher conductivity compared to distilled water. This is because some RO membranes may allow a small amount of minerals to pass through, which contributes to the water's ability to conduct electricity. The level of conductivity in RO water depends on the membrane type and the quality of the source water.
3) Organic Contaminants
Distillation: Distillation is a highly effective method for removing most volatile organic compounds (VOCs) from water. VOCs are organic chemicals that readily evaporate at room temperature. During the boiling process, these VOCs vaporize along with the water and are not condensed back into the purified water. This makes distillation a good choice for applications where the presence of organic contaminants can interfere with experiments.
RO Water: The effectiveness of RO in removing organic contaminants depends on the type of membrane used. Some RO membranes are not very effective at removing all types of organic molecules. Additionally, chlorine, a common disinfectant in tap water, can damage RO membranes. If your source water contains chlorine, a pre-treatment step like an activated carbon filter may be necessary to protect the membrane and ensure the effective removal of organic contaminants.
Now, let's examine how to choose between RO and distilled water based on the requirements of different lab experiments.
Choosing Between RO and Distilled Water for Specific Lab Applications
When selecting between RO and distilled water, it's essential to consider the specific requirements of each lab application. By understanding the unique properties of each type of water, lab personnel can ensure optimal conditions for their experiments:
Applications Where Distilled Water is Preferred
Distilled water is ideal for specific laboratory applications where exceptionally high purity is required. Here are some key situations where distilled water shines:
- Experiments requiring extremely low conductivity: Distillation removes nearly all ionized impurities from water, leading to very low electrical conductivity. This is crucial for experiments that measure conductivity, as even tiny amounts of ions can significantly affect the results.
- When working with very sensitive biological materials potentially affected by trace minerals: Some biological experiments involve delicate materials like enzymes or proteins. Trace minerals present in RO water, even at low levels, can interact with these materials and alter their behavior. Distilled water minimizes this risk by removing most minerals.
Applications Where RO Water May Be Suitable
For many routine laboratory tasks, RO water can be a viable and cost-effective alternative to distilled water. Here are some scenarios where RO is a good fit:
- Most routine laboratory applications where high purity is needed but trace minerals are not critical: Many lab procedures require purified water, but the presence of a few remaining minerals may not significantly impact the experiment. In these cases, RO water's sufficient purity makes it a good choice.
- When consistent water quality is important, as RO systems provide a continuous flow: Distillation involves boiling water and collecting the vapor, which is a slower process compared to RO. RO systems, on the other hand, offer a continuous flow of purified water, making them ideal for applications requiring a constant supply.
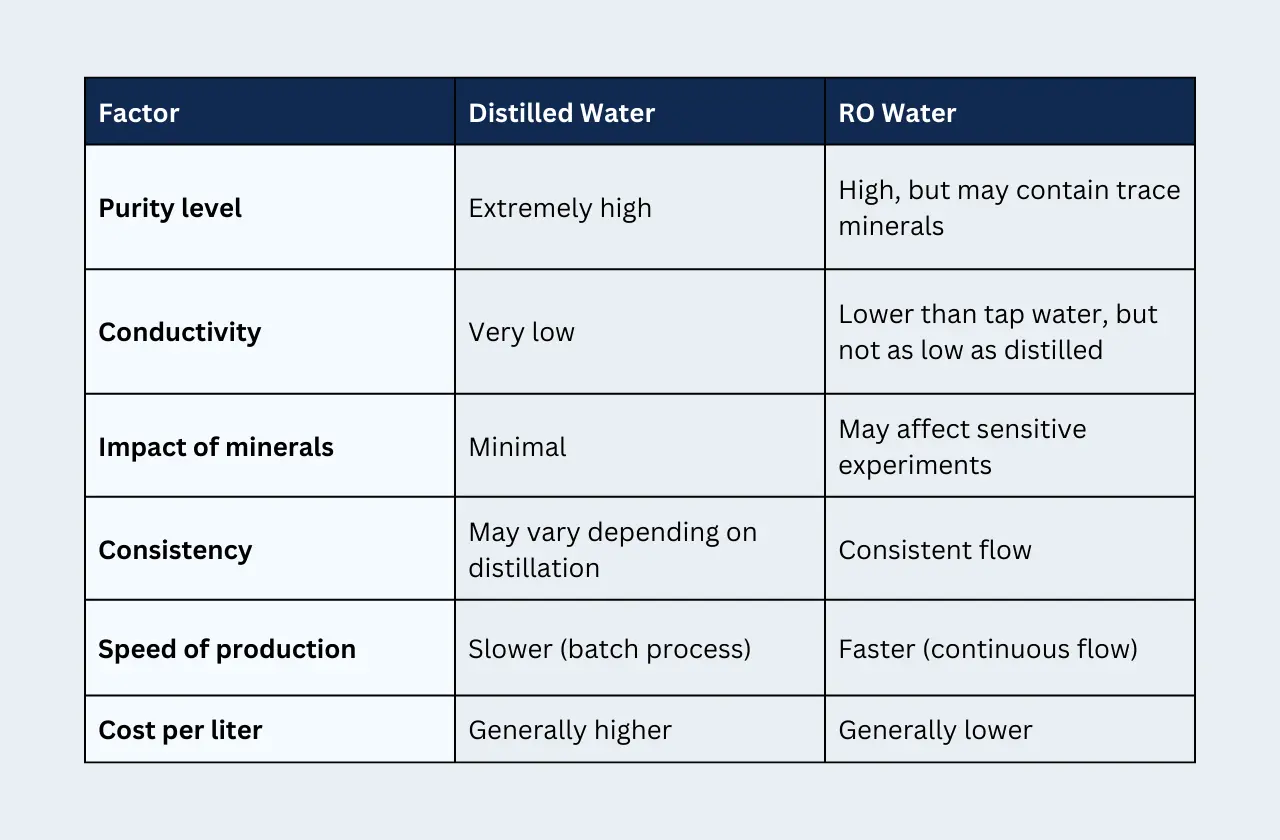
Here's a table summarizing the key considerations for choosing between RO and distilled water:
Conclusion
In conclusion, RO water and distilled water differ in their purification processes and mineral content. RO water undergoes reverse osmosis, removing impurities but retaining minerals. Distilled water, on the other hand, is produced by vaporizing water and condensing the steam, leaving impurities behind.
For laboratory use, the choice between the two depends on the specific application. Distilled water is often preferred for precise experiments requiring ultra-pure water, while RO water may suffice for general purposes. Understanding these distinctions ensures appropriate selection for experiments, maintaining accuracy and reliability in chemical analyses.
FAQs related to RO vs Distilled Water
1) Can RO water be used in place of distilled water?
In many cases, yes. RO water removes a wide range of impurities, including minerals, salts, and some bacteria. This makes it suitable for most lab uses where pure water is needed. However, there are exceptions. Some tests might require specific minerals present in distilled water. Always check your experiment's requirements before substituting.
2) Can I use RO water instead of distilled in a humidifier?
Generally, RO water is safe for humidifiers. It lacks minerals that can leave white dust, a common problem with tap water. However, some humidifier models might recommend using distilled water specifically. Consult your humidifier's manual for the best option.
3) Can I use RO water in my CPAP machine?
While RO water removes impurities, it might still contain microscopic particles. These can damage the CPAP machine's delicate components. Distilled water is preferred for CPAP machines because it ensures the absence of any minerals or particles.
4) What is a substitute for distilled water in a CPAP machine?
If distilled water isn't readily available, commercially bottled sterile water is an acceptable alternative for CPAP machines. However, avoid using tap water or RO water as they can damage the machine.
5) What is the alternative to distilled water?
For most lab applications requiring pure water, RO water is a suitable alternative to distilled water. Its advantage lies in its lower cost and continuous production. However, if your experiment demands the complete absence of minerals or requires specific minerals to be present, distilled water remains the better choice.
Latest Blogs