Stay up-to date on the
latest blogs. Join our
newsletter today!
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
What Are The Classes Of Fire: An Essential Guide

Written by Team Ryze Chemie
10 mins read · Jun 01, 2024

Lab work with chemicals often involves fire hazards. This is a serious problem as even small fires can cause harm and damage to people and property. We understand your concerns about safety in the laboratory. We want you to have the right knowledge to handle fire emergencies effectively.
In this article, we will discuss the different classes of fire, the types of fire extinguishers to use for each class, and other important safety tips. This will be a quick but essential guide for all laboratory personnel who work with chemicals.
What are the Classes Of Fire
Fire safety is crucial in any lab setting. Knowing the different classes of fires and how to address them can be the difference between a minor incident and a disaster. Let's break down the key fire types you might encounter in your work:

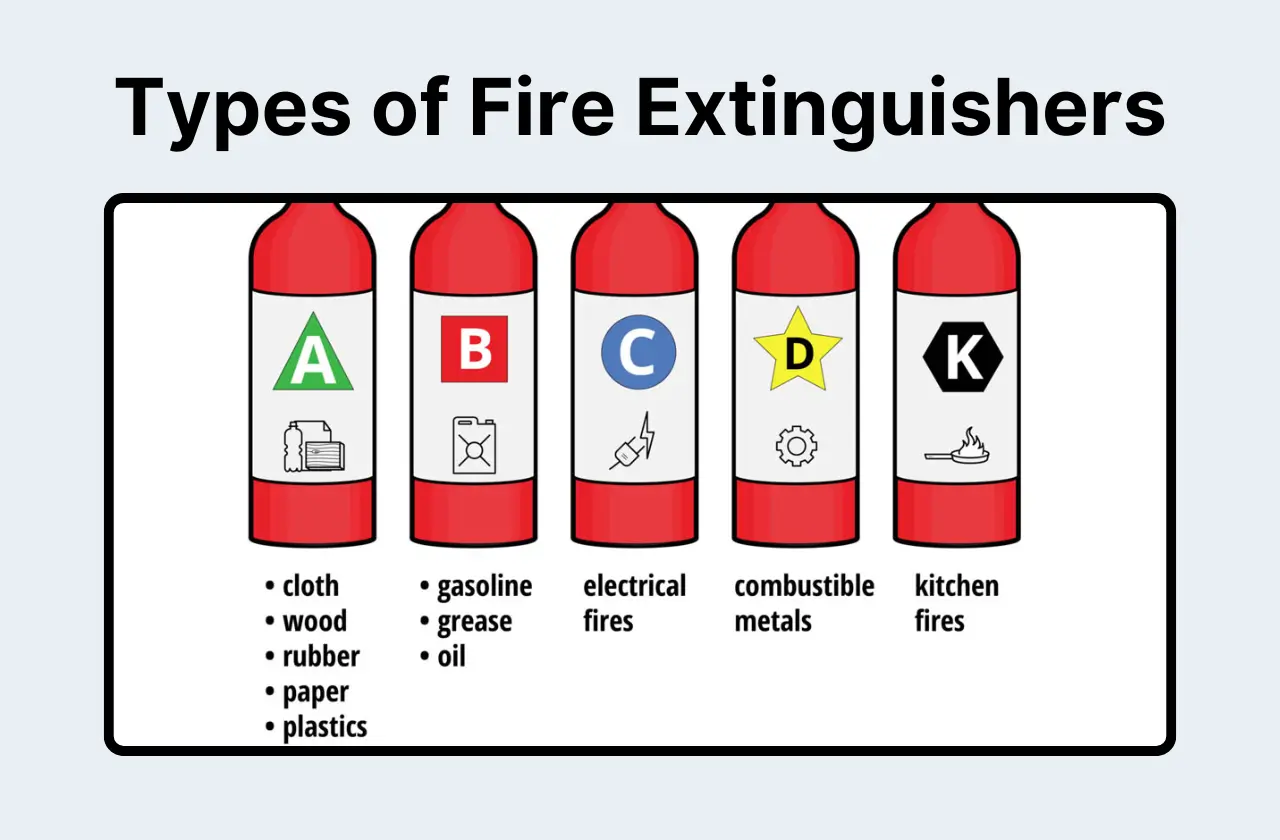
Class A Fires (Ordinary Combustibles)
- What they are: These are the everyday fires you're most likely familiar with. They involve things like paper, wood, cloth, and even some plastics.
- Where you'll find them: Think of your office space, break rooms, any areas where you store paper records or supplies.
- How to put them out: Good old water works great for these. Foam extinguishers or dry chemical ones can also be used.
Class B Fires (Flammable Liquids)
- What they are: These are the fires fueled by the liquids you work with regularly: solvents, oils, gasoline, alcohols – anything flammable in liquid form.
- Where you'll find them: Given the nature of lab work, these are unfortunately very common. Anywhere solvents are used or stored is a potential risk area.
- How to put them out: Avoid water! It can spread the fire. Use dry chemical extinguishers, carbon dioxide (CO2), or foam.
Class C Fires (Electrical)
- What they are: Any fire that starts from electrical equipment or wiring falls into this category.
- Where you'll find them: Think of all the electrical instruments, heating mantles, and other gear you use daily. Electrical issues can spark a fire quickly.
- How to put them out: Safety first! Cut the power if you can. Use a CO2 or dry chemical extinguisher (make sure it's non-conductive). Never use water on an electrical fire.
Class D Fires (Combustible Metals)
- What they are: These fires involve reactive metals like magnesium, sodium, potassium, and lithium.
- Where you'll find them: If your work involves these metals or their compounds, be extra cautious. Even small amounts can ignite.
- How to put them out: This is where special equipment is needed. Look for dry powder extinguishers made specifically for metal fires (they'll often say "Met-L-X" or similar). Never use water or standard extinguishers – it can make the fire worse!
Class K Fires (Kitchen/Cooking Oils)
- What they are: These involve cooking oils and fats, the kind you might find in a break room kitchen.
- Where you'll find them: Any kitchen area in your lab is a potential site for these fires if cooking equipment is present.
- How to put them out: Wet chemical extinguishers are your best bet. They cool the fire and create a barrier to stop it from spreading.
Knowing the fire class is important, but it's only the first step. Let's talk about the tools you need to fight fires.
Fire Extinguishers for Laboratories
Fire is a constant risk in laboratories. We work with flammable chemicals and heat sources daily. Fire extinguishers are essential tools to keep us safe. This section explains the different types of extinguishers, how to choose the right ones, and where to place them in your lab:
Types of Fire Extinguishers
Different fires need different extinguishers. Here's a quick overview:
- Class A: For ordinary fires like wood, paper, cloth. These are usually silver.
- Class B: For flammable liquids like solvents and oils. These are often red.
- Class C: For electrical fires. These are often blue.
- Class D: For combustible metals. These are usually yellow.
- Class K: For kitchen fires involving cooking oils and fats. These are often black.
Important Note: Some extinguishers can handle multiple classes of fires. These are often labeled as "AB," "BC," or "ABC."
Choosing the Right Extinguisher for Your Lab
Choosing the right extinguisher depends on what you have in your lab. Here are some common recommendations:
- Chemistry Labs: ABC dry chemical extinguishers are a good choice. They can handle most fires you might encounter.
- Biology Labs: ABC extinguishers are also good here. CO2 extinguishers are useful for electrical equipment fires.
- Physics Labs: CO2 extinguishers are a good choice for electrical equipment. Dry powder extinguishers are useful for metal fires.
Tip: Talk to your safety officer or fire marshal for specific recommendations for your lab.
Placement and Accessibility
Having the right extinguishers is important, but they won't help if you can't find them quickly.
- Place extinguishers near exits and work areas. This makes them easy to reach in an emergency.
- Keep extinguishers visible and unobstructed. Don't hide them behind equipment or in corners.
- Check extinguishers regularly. Make sure they are charged and in good working order.
- Train everyone in the lab on how to use extinguishers. Practice makes perfect.
Remember: Fire extinguishers are only for small fires. If the fire is too large, evacuate the building and call the fire department.
Fire extinguishers are great, but they're not the only way to stay safe. Let's cover some specific tips for fire safety in a lab setting.
Laboratory-Specific Fire Safety Tips
Fires in laboratories can be devastating. They can harm people, damage equipment, and destroy valuable research. As a chemical professional, you know that prevention is key. Here are specific tips to help you maintain a safe lab environment.
1) Chemical Storage:
Chemicals are the lifeblood of your research, but they can also be a fire hazard if not stored properly.
- Separate and Conquer: Flammable chemicals must live apart from reactive ones. Incompatibles should never share the same space.
- Label with Care: Each container needs a clear, accurate label. This includes the chemical name, hazards, and date received.
- Store in Approved Cabinets: Flammable cabinets are your friends. They keep flammables contained and protect them from accidental ignition.
- Limit Quantities: Only keep the amount of chemicals you need for immediate work. Excess chemicals increase the risk of fire.
2) Electrical Safety:
Electricity powers your lab, but faulty wiring and overloaded circuits can spark a fire.
- Ground Everything: Ensure all electrical equipment is properly grounded to prevent static buildup and electrical shocks.
- Protect from Overloads: Use circuit breakers and fuses to protect against electrical overloads, which can overheat wires and cause fires.
- Regular Maintenance: Have a qualified electrician inspect and maintain electrical equipment regularly. Don't try to fix electrical problems yourself.
3) Ventilation:
Proper ventilation keeps the air in your lab clean and prevents the buildup of flammable vapors.
- Fume Hoods for the Win: Use fume hoods when working with volatile chemicals. They contain vapors and expel them safely.
- General Ventilation: Ensure your lab has adequate general ventilation to remove fumes and maintain good air quality.
- Check Exhaust Systems: Regularly inspect and maintain exhaust systems to ensure they are functioning properly.
4) Fire Drills and Training:
Regular fire drills and training are essential for everyone in the lab.
- Know the Plan: Make sure everyone knows the lab's fire escape plan and their role in an emergency.
- Practice Regularly: Conduct fire drills at least twice a year to keep everyone prepared.
- Hands-on Training: Provide hands-on training for using fire extinguishers and other emergency equipment.
5) Emergency Procedures:
In the event of a fire, a quick and organized response is crucial.
- Sound the Alarm: Immediately activate the fire alarm if you discover a fire.
- Evacuate Safely: Follow the designated escape routes and evacuate the building calmly.
- Call for Help: Call the fire department or emergency services as soon as you are in a safe location.
- Do Not Re-enter: Do not re-enter the building until it has been declared safe by the authorities.
Sometimes the best way to learn is from others' experiences. Let's look at some real-life examples of lab fires and what we can learn from them.
Real-World Examples
Laboratory fires are more than a safety concern. They can disrupt research, damage expensive equipment, and cause injuries. Understanding the basics of fire chemistry and classification can be the key to preventing and controlling these incidents. Let's look at two real-world examples:
Case Study 1: The Ether Explosion
What Happened:
In a university organic chemistry lab, a researcher was distilling diethyl ether, a common solvent. The ether had been stored for a long time and had formed peroxides, unstable compounds that are highly explosive. When the distillation reached a high enough temperature, the peroxides detonated, causing a large explosion that shattered glassware and started a fire.

How Fire Classification Could Have Helped:
Diethyl ether is a Class B flammable liquid. Recognizing this, the researcher could have taken several precautions:
1) Proper Storage: Ether should be stored in airtight containers in a cool, dark place, and tested regularly for peroxides.
2) Ventilation: Good ventilation would have helped disperse any ether vapors, reducing the risk of a fire spreading.
3) Fire Extinguisher: Having the correct type of fire extinguisher (Class B) readily available would have allowed for quick suppression of the initial fire.
Case Study 2: The Sodium Metal Incident
What Happened:
A graduate student was cleaning glassware with acetone after a reaction involving sodium metal. Sodium reacts violently with water, and trace amounts of water in the acetone led to a rapid exothermic reaction. The heat generated ignited the acetone, causing a fire that spread quickly throughout the lab bench.
How Fire Classification Could Have Helped:
Acetone is a Class B flammable liquid, while the reaction with sodium would be classified as a Class D metal fire. Understanding this distinction would have led to different safety measures:
1) Compatibility: Never mix sodium with organic solvents like acetone. Use a specialized solvent like mineral oil for cleaning sodium residues.
2) Fire Suppression: A Class D fire extinguisher (designed for metal fires) would have been more effective than the standard Class B extinguishers typically found in labs.
3) Emergency Procedures: Knowing the specific hazards of sodium would have allowed for quicker and more appropriate emergency response.
Fire Classes: A Quick Refresher
- Class A: Ordinary combustible materials (wood, paper, cloth)
- Class B: Flammable liquids and gasses (solvents, fuels)
- Class C: Energized electrical equipment
- Class D: Combustible metals (sodium, magnesium)
- Class K: Cooking oils and fats
Key Takeaway: Knowing the fire class of the chemicals and equipment you work with is essential for choosing the right safety measures and fire suppression tools.
Disclaimer: This information is for educational purposes and should not replace formal safety training or consultation with safety experts. Always refer to your institution's specific safety protocols and guidelines.
Conclusion
Understanding fire classes is crucial for lab safety. It's not just theory – it's the foundation for choosing the right extinguisher and responding effectively to a fire. Remember, a small fire can escalate quickly in a lab environment. Knowing the different classes empowers you to act decisively, protecting yourself, your colleagues, and your research. Stay vigilant, prioritize safety, and make fire prevention a daily habit in your lab.
Latest Blogs